Diamonds, with their dazzling brilliance and enduring allure, are among nature’s most fascinating creations. These remarkable gems are more than just symbols of elegance and status—they are geological marvels with a story that stretches back billions of years. To truly appreciate the magnificence of diamonds, one must delve into their scientific roots and the extraordinary processes that give rise to their existence.
The Formation of Diamonds: A Journey Deep Within the Earth
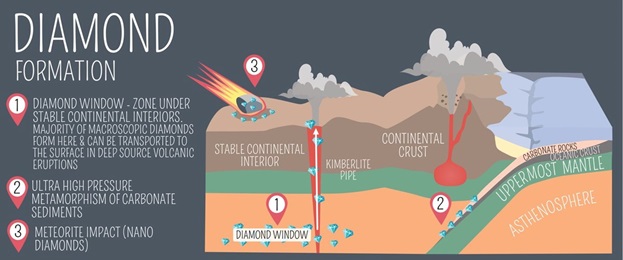
Diamonds are born under extreme conditions, far beneath the Earth’s surface. Approximately 150 to 200 kilometers below the crust, in the mantle, a combination of high pressure (about 725,000 pounds per square inch) and temperatures exceeding 1,000 degrees Celsius creates the perfect environment for carbon atoms to crystallize into diamonds.
The raw material for diamonds is carbon, the same element found in coal, graphite, and living organisms. However, the unique atomic arrangement of carbon in diamonds distinguishes them from all other carbon-based substances. Under intense pressure and heat, carbon atoms bond in a tetrahedral structure, resulting in an unparalleled hardness and optical brilliance.
The process, though, doesn’t end in the mantle. For diamonds to reach the surface, they must hitch a ride on volcanic eruptions. These eruptions create kimberlite pipes—vertical conduits through which molten rock propels diamonds upward, depositing them in the Earth’s crust where they can later be mined.
The Geological Timeline: Billions of Years in the Making
The formation of diamonds is not a quick process. Most natural diamonds were formed over 1 billion to 3.5 billion years ago. These timeframes provide a glimpse into the Earth’s ancient past, offering scientists clues about its evolution and conditions during different geological eras.
In addition to diamonds formed deep in the Earth, a small subset known as “impact diamonds” is created when meteorites strike the planet. The intense heat and pressure generated by the impact can cause carbon in the target rock to crystallize into diamonds. Though often smaller and less gem-quality, these diamonds are equally remarkable for their extraterrestrial origin.
Unraveling Diamond Characteristics Through Science
Diamonds owe their unique properties to their atomic structure and formation process. One of their most notable features is their hardness, ranking 10 on the Mohs scale. This makes diamonds the hardest natural substance on Earth, capable of scratching any material, including other diamonds.
Their optical properties are equally fascinating. Diamonds have a high refractive index and dispersion, allowing them to bend and split light into its constituent colors, creating the iconic “fire” that makes them so visually captivating.
Modern gemology uses advanced scientific techniques to analyze diamonds. Tools like spectroscopy and X-ray fluorescence help determine their origin, clarity, and whether they are natural or synthetic.
The Role of Kimberlite and Lamproite in Diamond Mining
Kimberlite and lamproite rocks are the primary hosts for diamonds. These rocks, named after regions where they were first discovered, form the conduits through which diamonds ascend during volcanic eruptions.
Kimberlite pipes, often shaped like a carrot, are rich in minerals and provide a concentrated source of diamonds. Once a kimberlite pipe is identified, extensive mining operations extract the diamonds embedded within.
Lamproite deposits, though less common, are equally significant. The Argyle Diamond Mine in Australia, famous for its rare pink diamonds, is a notable example of a lamproite deposit.
The Science Behind Lab-Grown Diamonds
In recent years, advancements in technology have made it possible to replicate the natural diamond-forming process in laboratories. Lab-grown diamonds, also known as synthetic diamonds, are created using one of two methods: High-Pressure High-Temperature (HPHT) or Chemical Vapor Deposition (CVD).
- HPHT Method: This technique mimics the high-pressure and high-temperature conditions of the Earth’s mantle. A small diamond seed is placed in a chamber with carbon, subjected to intense pressure and heat, and allowed to grow layer by layer.
- CVD Method: In this process, a carbon-rich gas is heated to produce plasma. The carbon atoms from the gas then deposit onto a diamond seed, gradually forming a crystal.
These lab-grown diamonds possess the same physical, chemical, and optical properties as natural diamonds, making them nearly indistinguishable to the naked eye.
The Ethical and Environmental Dimensions
The scientific study of diamonds also highlights the ethical and environmental challenges associated with their mining. Diamond extraction can disrupt ecosystems and contribute to deforestation, soil erosion, and pollution. In addition, the history of conflict diamonds—gems mined in war zones and used to finance armed conflicts—has raised significant ethical concerns.
To address these issues, initiatives like the Kimberley Process have been established to ensure that diamonds are sourced responsibly. Lab-grown diamonds offer a sustainable alternative, reducing the environmental impact and eliminating the risk of conflict-associated origins.
The Fascination with Rare Diamonds
Beyond common diamonds, the world is captivated by rare and unique specimens that exhibit extraordinary qualities. Fancy-colored diamonds, such as vivid blues, pinks, and yellows, are highly sought after. Their colors arise from trace elements or structural anomalies:
- Blue Diamonds: Colored by the presence of boron.
- Pink Diamonds: The result of stress-related distortions in the crystal lattice.
- Yellow Diamonds: Caused by nitrogen impurities.
These rare gems often command astronomical prices, reflecting their scarcity and desirability.
The Future of Diamond Research
Ongoing scientific research into diamonds continues to unveil their potential beyond adornment. Diamonds’ thermal conductivity, resistance to wear, and optical clarity make them ideal for industrial applications. They are used in cutting tools, semiconductors, and even quantum computing.
In medicine, diamond coatings are being explored for surgical tools, while their biocompatibility opens avenues for drug delivery and implants. These innovations illustrate how diamonds are transcending their traditional role to become integral to technological advancements.
Conclusion
The journey of diamonds, from their formation deep within the Earth to their transformation into timeless treasures, is a testament to the marvels of nature and human ingenuity. These gems encapsulate billions of years of geological history, embodying the interplay of extreme conditions and elemental beauty.
As science continues to explore the mysteries of diamonds, their significance extends beyond their aesthetic appeal, revealing their role in technology, sustainability, and human progress. Truly, diamonds are more than just objects of desire—they are a window into the wonders of our world and its boundless potential.
